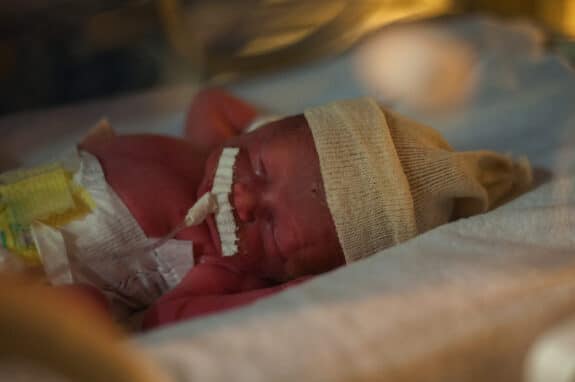
The U.S. Food and Drug Administration (FDA) is taking steps to protect public health by addressing the potential risks posed by probiotic products containing live bacteria or yeast, commonly known as probiotics, to preterm infants in hospital settings. The FDA has sent a letter to healthcare providers to raise awareness about this issue and has issued warning letters to companies that illegally sell these products for treating or preventing certain diseases in preterm infants.
Probiotic products, often marketed as foods or dietary supplements, contain live organisms like bacteria or yeast. The FDA is concerned because these products can be harmful to preterm infants and are being unlawfully sold to treat or prevent diseases in hospital settings, such as reducing the risk of necrotizing enterocolitis. Preterm infants given probiotics are at risk of invasive, potentially fatal infections caused by the bacteria or yeast in these products.
The FDA is aware that certain probiotics used in hospitals to prevent necrotizing enterocolitis have caused invasive diseases, including one infant death in 2023, and have been linked to over two dozen other reported adverse events in the United States since 2018. The agency is investigating reports suggesting that these products may have contributed to additional adverse events, including deaths, and is actively gathering evidence and medical records. Any death or adverse event in an infant following the use of probiotic products is deeply concerning, and the FDA is working closely with healthcare providers to understand the connection between these products and the reported adverse events in preterm infants.
It is important to note that the FDA has not approved any probiotic product as a drug or biological product for infants of any age. Probiotics that are unapproved and unlicensed, used to treat or prevent diseases in preterm infants, have not undergone the FDA’s comprehensive evaluation for safety and effectiveness. Moreover, they have not been assessed for compliance with the agency’s strict manufacturing and testing standards, including testing for other organisms. To be legally marketed as drugs and biological products, these products require approval through a Biologics License Application to ensure proper evaluation. In the absence of an approved product, healthcare providers who administer probiotic products to treat, mitigate, cure or prevent diseases or conditions must submit an Investigational New Drug application to the FDA to ensure the use of unapproved products is conducted safely.
The FDA says they are committed to addressing violations and safety issues associated with these products and holding manufacturers accountable. The agency has issued a warning letter to Abbott Laboratories for its product, Similac Probiotic Tri-Blend, containing B. infantis (Bb-02), S. thermophilus (TH-4) and B. lactis (BB-12). It is important to clarify that this product is not an infant formula and is not related to the previous issues with powdered infant formula from Abbott Nutrition. Abbott has agreed to discontinue sales of the Similac Probiotic Tri-Blend and is cooperating with the FDA to implement further corrective actions.
In the warning letter, it is highlighted that Abbott sells the probiotic product for use in hospital settings for preterm infants. According to the company’s websites and marketing materials, the product is an unapproved new drug and an unlicensed biological product, violating the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Public Health Service Act. Additionally, when intended for consumption by preterm infants, the Bb-02 and TH-4 ingredients do not meet the applicable safety requirements, making the product an adulterated dietary supplement under the FD&C Act.
The FDA has also issued a warning letter to Infinant Health, Inc (formerly Evolve BioSystems Inc.) concerning its probiotic product, Evivo with MCT Oil, which is an unapproved and unlicensed product sold for treating or preventing disease in preterm infants, in violation of the FD&C Act and the Public Health Service Act. The product was intended to be added to food for preterm infants and was found to be an adulterated food under the FD&C Act. This product has since been voluntarily recalled and is no longer available in the U.S.
The FDA is taking action to address the potential risks associated with probiotic products and preterm infants, ensuring the safety and well-being of this vulnerable population. The agency’s warning letters and investigations aim to hold companies accountable for unlawfully selling these products and to gather the necessary evidence to better understand any adverse events reported in preterm infants.
Related Articles: